MechanoUrology' Engineering, Biomechanics, Biochemistry and Urology
University
Goals
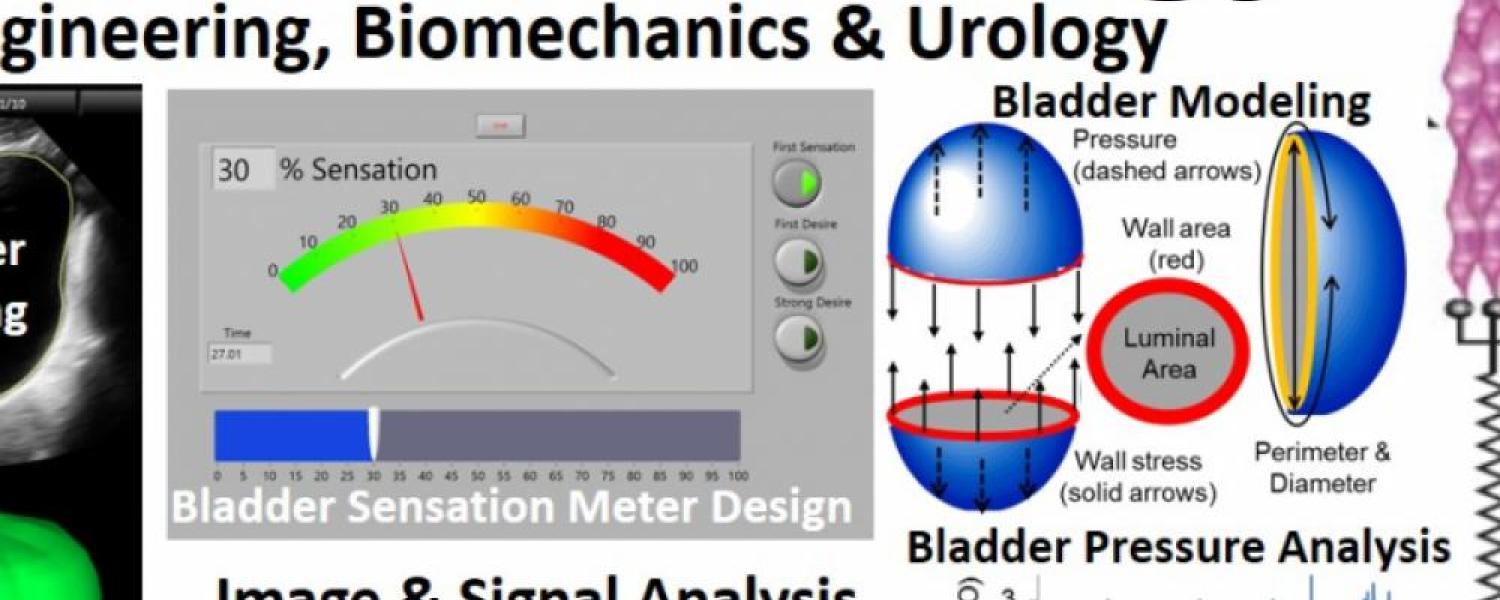
Overactive bladder (OAB) occurs during bladder filling and affects ~20% of the adult US population. The current tool for evaluating bladder filling is a urodynamics study which uses a catheter to fill the bladder while pressure is measured. Tension sensitive nerves in the bladder wall are responsible for providing bladder fullness information to the brain and increased bladder wall tension during filling is thought to be a critical factor in OAB. However, pressure often increases little during bladder filling and does not accurately reflect changes in bladder wall tension. Therefore, effective assessment of OAB using standard clinical urodynamics testing is difficult or impossible, and a new diagnostic test for OAB that includes the evaluation of bladder wall tension is needed. In addition to pressure, the biomechanical parameters that can directly affect the load on the bladder wall tension sensors during filling include bladder geometry, acute changes in bladder elasticity, and spontaneous rhythmic bladder contractions. Our team has discovered that the bladder is a smart material that can acutely regulate its preload tension, and we have clinically quantified this “dynamic elasticity” in patients with OAB.
The team will be working to develop improved clinical biomechanical diagnostics for OAB and other bladder disorder and to understand the complex biomechanical and biochemical mechanisms responsible for dynamic elasticity in humans and animal models of OAB.
Issues
Porjects will include:
-Develop algorithms for analysis of clinical urodynamics data
-Analyze ultrasound images and develop algorithms to calculate novel clinical bladder biomechanics parameters relevant to OAB & correlate these parameters with patient-reported sensation changes
-Develop algorithms to analyze rhythmic bladder contractions and pressure fluctuations and correlate with sensation changes
-Develop algorithms to analyze bladder ultrasound video cines to identify micromotion of the bladder wall
-Analyze bladder sensation parameters and develop algorithms to streamline this process
-Develop novel biomechanical models of bladder & smooth muscle
-Perform experiments to determine the effects of limited blood flow and ischemia in isolated animal bladders
-Perform biochemical analyzes to determine molecular mechanisms responsible for bladder biomechanical behavior
-Develop algorithms for analysis of clinical urodynamics data
-Analyze ultrasound images and develop algorithms to calculate novel clinical bladder biomechanics parameters relevant to OAB & correlate these parameters with patient-reported sensation changes
-Develop algorithms to analyze rhythmic bladder contractions and pressure fluctuations and correlate with sensation changes
-Develop algorithms to analyze bladder ultrasound video cines to identify micromotion of the bladder wall
-Analyze bladder sensation parameters and develop algorithms to streamline this process
-Develop novel biomechanical models of bladder & smooth muscle
-Perform experiments to determine the effects of limited blood flow and ischemia in isolated animal bladders
-Perform biochemical analyzes to determine molecular mechanisms responsible for bladder biomechanical behavior
Desired Majors
Mechanical Engineering
Biomedical Engineering
Electrical and Computer Engineering
Chemical and Life-Science Engineering